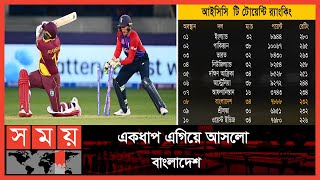
An industrial fuel, 'water gas', which consists of a mixture of \( ...
Published at : October 08, 2022
An industrial fuel, 'water gas', which consists of a
mixture of \( \mathrm{H}_{2} \) and \( \mathrm{CO} \) can be made by passing steam over red-hot carbon. The reaction is
\[
\mathrm{C}(\mathrm{s})+\mathrm{H}_{2} \mathrm{O}(\mathrm{g}) \rightleftharpoons \mathrm{CO}(\mathrm{g})+\mathrm{H}_{2}(\mathrm{~g}), \Delta \mathrm{H}=+131 \mathrm{~kJ}
\]
The yield of \( \mathrm{CO} \) and \( \mathrm{H}_{2} \) at equilibrium would be shifted to the product side by:
(A) raising the relative pressure of the steam
(B) adding hot carbon
(C) raising the temperature
(D) reducing the volume of the system
hysics wollah
Activate Windows
Go to Settings to
📲PW App Link - https://bit.ly/YTAI_PWAP
🌐PW Website - https://www.pw.live
mixture of \( \mathrm{H}_{2} \) and \( \mathrm{CO} \) can be made by passing steam over red-hot carbon. The reaction is
\[
\mathrm{C}(\mathrm{s})+\mathrm{H}_{2} \mathrm{O}(\mathrm{g}) \rightleftharpoons \mathrm{CO}(\mathrm{g})+\mathrm{H}_{2}(\mathrm{~g}), \Delta \mathrm{H}=+131 \mathrm{~kJ}
\]
The yield of \( \mathrm{CO} \) and \( \mathrm{H}_{2} \) at equilibrium would be shifted to the product side by:
(A) raising the relative pressure of the steam
(B) adding hot carbon
(C) raising the temperature
(D) reducing the volume of the system
hysics wollah
Activate Windows
Go to Settings to
📲PW App Link - https://bit.ly/YTAI_PWAP
🌐PW Website - https://www.pw.live

pw